We aim to establish a universal understanding of chemical processes and phenomena involving molecules by independently developing molecular level theories with high generality and applying them to real systems.
Development of new methods in theoretical chemistry
Although each molecule can be understood by quantum chemistry, when countless molecules gather, the property as a group appears.
There is a hierarchical structure in nature, and a comprehensive viewpoint is important for understanding chemical phenomena.
Hybrids of quantum chemistry and statistical mechanics
Most chemical reactions occur in solvents. However, despite the fact that such solvent effect is a crucial key to reactivity, the theory dealing with it is still not well developed. The theory, RISM-SCF-SEDD method, that integrates quantum chemistry with the integral theory of numerator liquid developed in this laboratory is a powerful method to approach the essence of the chemical process of molecules in solution. Besides this, we develop and apply new theory which is not caught only by the framework of conventional electronic state theory, and analyze realistic reaction.- Examples;
- RISM-SCF-SEDD
- 3D-RISM-SCF
Statistical mechanics for polyatomic molecular systems
Chemistry is aimed at a group of molecules, and the viewpoint of statistical mechanics is important. From a viewpoint different from widely popular molecular simulation (molecular dynamics method, Monte Carlo method), we are developing a new statistical mechanics theory which directly describes solvation structure and structural fluctuation at molecular level. Furthermore, it is applied to large scale systems including liquids and biomolecular system using these developed methods.- Examples;
- MC-MOZ
- Flexible-RISM
Application to actual chemical reactions and processes
The actual chemical reaction / chemical process is complicated.
By utilizing various theories such as statistical mechanics and dynamics as well as quantum chemistry, molecular level understanding based on the first principle becomes possible.
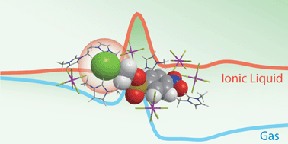
In particular, by utilizing the theory independently developed in the laboratory, phenomena occurring in all kinds of solutions such as organic solvents and aqueous solutions are targeted. There are many examples that realized first-principles calculation in the world for the first time, such as chemical reaction in supercritical fluid and ionic liquid, calculation of pKa.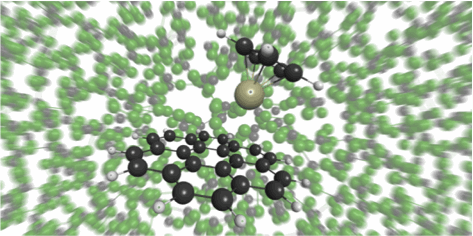
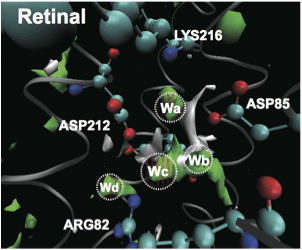 Based on the statistical mechanics theory of molecules, we derive molecular level information such as hydration structure inside proteins. Furthermore, by combining with the peripheral theory including quantum chemistry theory, we aim to establish a new viewpoint which could not be obtained by the conventional method.
Based on the statistical mechanics theory of molecules, we derive molecular level information such as hydration structure inside proteins. Furthermore, by combining with the peripheral theory including quantum chemistry theory, we aim to establish a new viewpoint which could not be obtained by the conventional method.
Chemical reactions
Computational chemistry is a powerful means of directly seeing the whole picture of a reaction. By utilizing the existing theory of electronic state as well as the theory developed independently, we clarify the essence of various chemical reactions such as reaction in solution and catalytic reaction.
In particular, by utilizing the theory independently developed in the laboratory, phenomena occurring in all kinds of solutions such as organic solvents and aqueous solutions are targeted. There are many examples that realized first-principles calculation in the world for the first time, such as chemical reaction in supercritical fluid and ionic liquid, calculation of pKa.

Biomolecular systems
 Based on the statistical mechanics theory of molecules, we derive molecular level information such as hydration structure inside proteins. Furthermore, by combining with the peripheral theory including quantum chemistry theory, we aim to establish a new viewpoint which could not be obtained by the conventional method.
Based on the statistical mechanics theory of molecules, we derive molecular level information such as hydration structure inside proteins. Furthermore, by combining with the peripheral theory including quantum chemistry theory, we aim to establish a new viewpoint which could not be obtained by the conventional method.